Predominantly, pure chemicals can exist in different states of aggregation depending on prevailing pressure and temperature conditions. The figure shows the phase diagram of carbon dioxide (CO2). By virtue of this diagram, it can be inferred which state of matter exists at a certain pressure and temperature. Principaly, there are four distinct regions where matter occurs as a single phase: solid(s), liquid (l), gas (g), and supercritical (sc). In this circumstance, pressure and temperature can be changed without phase transition. These areas are limited to lines where the compound is in equilibrium with two different phases.
The sublimation curve represents the pressure and temperature conditions in which solid and gaseous states exist simultaneously. The coexistence of solid and liquid phase is represented by the melting curve, while the evaporation curve shows the equilibrium of gas and liquid states. The intersection point of these three curves is called the triple point. Here matter is in equilibrium in solid, liquid and gaseous state.
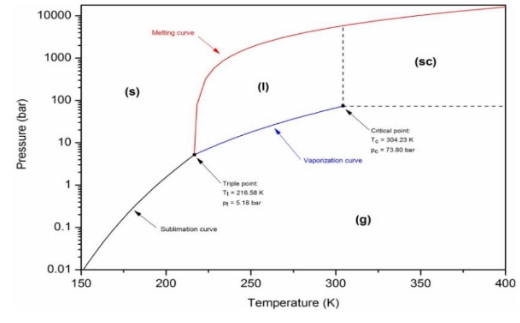
Figure 1- CO2 phase diagram
While the solvent in the supercritical phase has the ability to flow and scavenge like a liquid, it can dissolve and transport substances due to the polarity difference by diffusing into the cells in organic materials such as gas. In fact this process is the basic logic of extraction, and isolating materials that can be separated from substances by polarity differences.
Supercritical fluid extraction (SFE) is an intriguing alternative technique to conventional solid-liquid extraction with organic solvents. Among all supercritical fluids, the most common and suitable solvent in SFE is carbon dioxide. Foremost, carbon dioxide exhibits moderately critical conditions. The critical point of carbon dioxide is 31.1 °C critical temperature and 73.8 bar pressure.
Thermal degradation of natural compounds during the extraction process can be prevented correspondingly.
The advantages of extracting substances by supercritical extraction are as follows;
By the reason it is a completely closed system, no free oxygen radical formation is observed in the system, so the quality of the product obtained after the process is high.
The use of nonpolar solvents in the supercritical phase, increasing their polarity, and thus realizing the process without the need for sources such as heat or light, provides both energy and quality advantages.
As a consequence of the use of gases and especially carbon dioxide as a solvent in this process, no solvent residue is formed in the final product. On account of this, it is ensured that completely pure extracts are obtained.
Since it is a closed system, different gases or different modifying solvents can be used as support in addition to carbon dioxide.
Stevia rebaudiana (Bertoni) Supercritical Extraction Overview
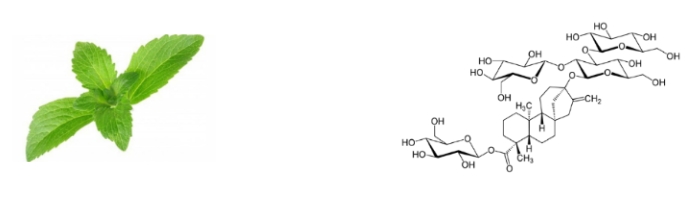
Figure 3- Stevia rebaudiana bertoni and Rebaudioside A molecular structure
Rebaudioside A is a steviol glycoside purified from the Stevia rebaudiana Bertoni plant belonging to the Asteraceae family, a plant species native to the South American region. The main components of the steviol glycosides in the stevia plant are stevioside (4-14%) and rebaudioside A (2-4%), and there is only one glucose molecule difference between them. Especially rebaudioside A, which is the most important of these steviosides, has an effect 250-300 times more sweetness than the sugar that is currently consumed. The cytotoxic effect of stevioside and rebaudioside A was studied in HepG2 cells as LDH activity and no cytotoxic effect was observed at concentrations of 0.5, 1.0 and 2.0 mg/mL. Steviol itself is an aglycone diterpene, while rebaudioside A and stevioside are glycoside-based structures with this aglycone carbon skeleton. Rebaudioside A and stevioside both contain a D-glucose group attached to the C19 carbon, and stevioside has an additional di-glycosyl group at the C13 carbon, while rebaudioside A has a tri-glycosyl sugar group. In fact, although steviol does not have any sweetening effect on its own, the sweetness potential increases with the increase in the attached glucose groups. The effect of the presence or absence of methylene double bond on the aglycone structure of steviol glycosides on the taste was investigated and it was determined that the presence of the methylene double bond in the aglycone structure was necessary for the presence of sweet taste.
The total steviol glycoside content of dried stevia leaves is around 18%, of which 4% is rebaudioside A and the remaining 14% is other steviol glycosides. That is, 20-22% of the total extracted glycosides are rebaudioside A and the remaining 78-80% are other steviol glycosides.
|
Process |
Operation Conditions |
|
Dehydrierte Steviablätter |
Ernte und Blatttrennung |
|
Extraktion |
Extraktion mit heißem (50-60oC) Wasser 2-3 Mal |
|
Verklumpen |
Hinzufügen von Calciumhydroxid und Aluminiumsulfat |
|
Filtration |
Filterpresse |
|
Absorption auf Harz |
Steviolglykoside werden an das Harz adsorbiert und dann mit Hilfe von Alkohol vom Harz getrennt. |
|
Destillation |
Alkoholverdunstung |
|
Ionenaustausch |
Kationen- und Anionenharzmischung |
|
Konzentration |
Wasserentfernung durch Vakuum |
|
Mikrofiltration |
1 Mikron |
|
Sterilisation |
120oC, 1 Minute |
|
Sprühtrockner |
Trockner |
In conventional methods, steviol glycoside production is carried out as in the above steps. Along with ethanol, methanol, which is considered toxic, is also used in this process. In the crystallization and purification of rebaudioside A, peculiarly this alcohol is used.
Apart from conventional methods, the use of supercritical extraction in the isolation of steviol glycosides has also been proven in studies. With maximum yield, steviol glycosides can be obtained as a mixture by using water modification in supercritical extraction, and in the next step, it is possible to obtain pure glycosides by decolorization and then water evaporation.
The biggest detriment of conventional methods is that they create heavy metals such as arsenic and lead. However, this heavy metal transport is not reflected in the extract in supercritical extraction.
Stevia leaves Supercritical Fluid Feasibility
Stevia leaves bulk density 540 kg/m3
SCF total extraction yield 18%
SCF extract Reb-A content 4%
|
Extractor Volume |
Liter |
2000 |
|
Raw material bulk density |
kg/m3 |
540 |
|
Amount of raw materials used in a single process |
Kg |
1080 |
|
Duration of process |
Hours |
2 |
|
Raw material particle size |
mm |
1-5 |
|
Total extract yield |
% |
18 |
|
Total Reb-A yield* |
% |
4 |
|
Daily process amount |
Unit |
10 |
|
Amount of raw materials used daily |
kg |
10800 |
|
Total amount of glycoside obtained per day |
kg |
1944 |
|
Total amount of Reb-A obtained daily |
kg |
432 |